As an entry from K. K. Jain’s Textbook Of Hyperbaric Medicine all chapter references refer to the 4th Edition.
Hypoxemia and ischemia are the underlying pathologies in many of the conditions seen in an emergency department. In addition to resuscitation and other emergency treatments, hyperbaric oxygen plays a vital role in the management of these patients. This topic is discussed under the following headings:
Indications for the Emergency Use of HBOT
Use of HBOT in Cardiopulmonary Resuscitation (CPR)
Case Studies of Medical Emergencies Treated with HBOT
Potential Future Uses of HBOT Therapy in Emergency Medicine
Introduction
Timely resuscitation by augmentation of oxygen delivery to tissue damaged by ischemia is key to many emergency medicine interventions in sickness and injury. Further, the prompt attempt to lessen reperfusion injury and necrosis after initial resuscitative clinical success should not be forgotten. Finally, the patient, once past the initial resuscitative effort, followed by restorative oxygenation, should receive maintenance oxygenation as needed to optimize the chance of continued recovery. In other words, one of the major purposes of an emergency department is to first assure proper oxygen delivery to many of its sick and injured. The oxygen delivery must be adjusted to maximize therapeutic effect in the emergency medicine interventional phases of resuscitation and restoration, and the maintenance phase of patient management.
Oxygen delivery to tissue is dependent on cardiac output. Oxygen delivery to tissue is given by the following formula:
Oxygen delivery = cardiac output x arterial oxygen content
(Shannon &Celli 1991)
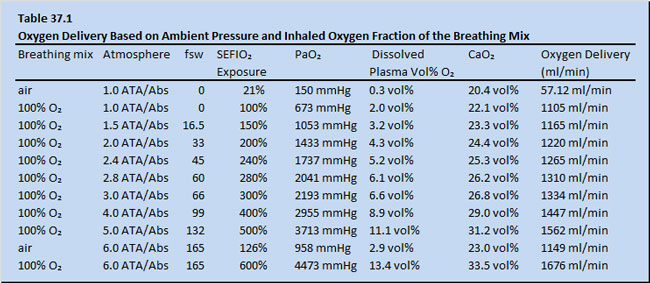
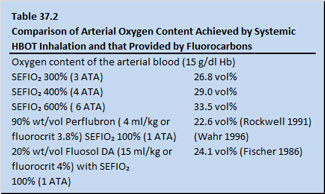 Table 37.1 shows data on a hypothetical adult possessing a hemoglobin level of 15 grams per decaliter. The assumption is made that the patient would have been placed by whole body exposure to differing barometric pressures with varying surface equivalent fraction of inspired oxygen (SEFIO2) . The potential oxygen delivery values assuming a cardiac output of 5 liters/min and an oxygen saturation of hemoglobin of 100% are calculated (Table 37.1). Note that the arterial oxygen content achieved by systemic HBOT inhalation is in the range of that provided by fluorocarbons (Table 37.2).
Table 37.1 shows data on a hypothetical adult possessing a hemoglobin level of 15 grams per decaliter. The assumption is made that the patient would have been placed by whole body exposure to differing barometric pressures with varying surface equivalent fraction of inspired oxygen (SEFIO2) . The potential oxygen delivery values assuming a cardiac output of 5 liters/min and an oxygen saturation of hemoglobin of 100% are calculated (Table 37.1). Note that the arterial oxygen content achieved by systemic HBOT inhalation is in the range of that provided by fluorocarbons (Table 37.2).
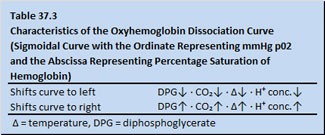
It is not certain that oxygen delivery to ischemic tissue always has the full benefit of 1.34 ml oxygen carriage per gram of hemoglobin. Hemoglobin’s release of oxygen to peripheral ischemic tissue is relative to plasma temperature, C0₂ content, hydrogen ion concentration and diphosphoglycerate levels (Table 37.3).
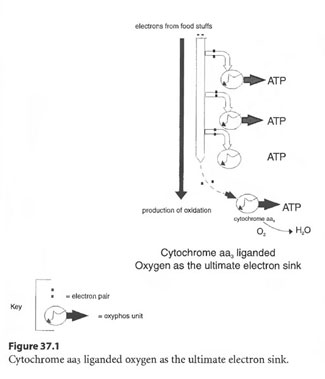
The dissolved oxygen in the plasma fraction is more independent of the above tabulated variables and more dependent upon ambient pressure. Oxygen, if successfully delivered to mitochondria, becomes a powerful electron acceptor as it attaches to the end of the cytochrome chain. Essentially, electrons, as they are stripped off the nutrient source of fats, carbohydrates and protein, plummet down an elegant cytochrome chain within mitochondria to a receptive oxygen diatomic molecule (O2 liganded to cytochrome aa3) as shown in Figure 37.1. As the electrons fall in energy level as they traverse down the cytochrome chain, energy is tapped to empower “Oxphos” units to make ATP. The ATP, among many things, runs membrane ion pumps (ionophores) to maintain acid base and electrolyte balance (deDuve 1984).
Non-delivery of oxygen wrecks the human machine functionally, then structurally, as evidenced by the almost instant loss of consciousness and neurologic damage which occurs by asphyxiating inhalation of 100% N2 or by inhalation of high levels of H2S, HCN or CO which bind to cytochrome aa3 stopping short the oxidative flow of electrons.
Indications for Emergency Use of HBOT
 Approved indications for HBOT which are considered to be an emergency are shown in Table 37.4.
Approved indications for HBOT which are considered to be an emergency are shown in Table 37.4.
Often the patient’s entry into the medical system for these conditions does not follow regular hours. As a result, these patients enter the system through the emergency department. Emergency medicine, especially in the United States, is a specialty brought into existence by public demand rather than preexistence of a discrete specialty of skills or knowledge.
In fact specialists are, more often than not, equally (if not more) skilled in the management of medical emergencies in their respective specialties. The number of emergency department visits has rather steadily increased in the United States despite the United States Federal Government’s effort to increase the number of primary care clinician by favorable reimbursement. The American public has voted in legislation giving the patient the right to determine a medical emergency (Prudent Lay Person Laws). The United States Federal Government has produced strong regulations (EMTALA = Emergency Medical Treatment and Active Labor Act) to direct hospitals and physicians to not shirk their duty of medical evaluation of all patients regardless of their ability to pay and regardless of the initial trivialness of a complaint when the patients present to an emergency department. Collectable reimbursements for emergency medical services, accordingly, range from 15 to 45% of charges, whereas other specialties in the U. S. often enjoy reimbursement of 45% to 90%. The reimbursement constraints and legal directives to see and treat all comers properly and effectively have placed rather amazing evolutionary operational stresses on emergency departments. Fast Tracks have been developed to move less complicated and less severe cases more quickly. Managed care, while initially accusing emergency medicine of being cost-ineffective, has embraced it with increasing enthusiasm, because the advantage of low marginal cost of seeing extra patients on a timely basis can be passed on to managed care.
Managed care’s reaction to emergency medicine might reflect some interesting features that are inherent to emergency medical services. Hospitals have slowly come to realize that emergency departments are in fact a front porch to the public. Many progressive hospitals have architecturally expanded and embellished their emergency departments. Many hospitals have enclosed or internalized these “front porches” to make the emergency department patient waiting room the de facto hospital lobby.

The marginal cost of seeing one more emergency patient in a day is extremely low. The emergency department is a technological work bench with a full range of equipment not available in a private office or clinic. Emergency departments have sophisticated risk management and quality management institutionally in place. The spectrum of clinical intervention ranges from the application of the Ottowa Rules (Stiell 1994) for economic fast evaluation of ankle sprains, all the way to the use HBOT in resuscitation of a CO-intoxicated patient on ventilator because of cardiopulmonary arrest. Procedural skills and equipment needed for resuscitators, once available only in non-emergency department areas of the hospital, have tended to become especially available in the emergency department. An example is the procedural skill of rapid sequence intubation (RSI) to protect a patient’s airway. This skill has moved from the exclusivity of the Anesthesia Department to being a routinely performed skill by emergency physicians in the emergency department. The same trend is just starting to merge for focus-limited diagnostic ultrasonography and HBOT therapy. Increasingly, emergency departments are being staffed by board-certified, residency-trained emergency physicians. Increasingly, emergency medicine residency programs are adopting residency training rotations in focus limited ultrasonography and fellowships in hyperbaric medicine.
The emergency department is open 24 hours a day and is legislated by popular demand to be egalitarian in kindness, skill and attention given to all patients at its door. Health care providers in the emergency department are increasingly in-serviced to be “Disneyesque” in demeanor by being a “health care cast,” offering respectful attentions to all patients. The recent development of two specialty units within the emergency department in the U.S. has added a curious dimension to emergency medical services. One of these – observation units- run by emergency departments have allowed serious or unclear patient cases to be managed aggressively with fast-moving diagnostics and interventions, within the first 24 hours of presentation to the hospital health care team. Again, managed care has picked up on the cost saving potential of this approach. The other – Hyperbaric Units – contained within emergency departments take advantage of the economy of having a doctor automatically present. The U. S. Center for Medicaid and Medicare Services (CMS) requires a physician to be physically present on site during a hyperbaric treatment to enable reimbursement for both Part B (physician services) and Part A (equipment, disposable medical supplies, technician, nursing service, and houseplant use).
Recently CMS has allowed reimbursement for the emergency physician to multitask for physician coverage of hyperbaric patient treatments for chambers in the emergency department. This is most fitting, for across its broad spectra of care, emergency medicine is in fact operationally a multitasking specialty in every respect.
The emergency use of HBOT often requires multiple treatments, both scheduled and unscheduled, as needed over 24 hours. The patient condition can be clinically static, e.g., the management of a diabetic foot wound. At other times treatments can be dynamically complicated, e.g., the critical care of a ventilator-supported patient with Fournier’s gangrene who is being staged for serial debridement operations. Again, traditionally, the emergency physician is at comfort with treating stable, non-urgent patients alongside severely unstable and complicated cases.
Finally, issues of continuity of care are rapidly improving in emergency medicine. With electronically recorded medical information, emergency medicine is moving from episodic, disconnected care to medical intervention, informationally connected to both past and future primary and specialty medical care on any one patient. In fact, emergency medicine is fast becoming a vital link in American primary medical care. In the same way, emergency department based hyperbaric medicine units have utilized electronic patient records to include electronically recorded wound pictures and treatment plans to maintain consistency in hyperbaric patient care from shift to shift.
 In the United States, the prehospital emergency medical base station medical control is well worked out using the “911” entry into the hyperbaric medical system of the country by calling the Divers Alert Network (DAN) hot line. It is intended that HBOT be used in planned, controlled, and prospective human trials in stroke intervention in the United States. These stroke trials will utilize a similar “call 911 stroke” emergency medical service (EMS) telephone number. EMS HBOT therapy will be discussed further in the section on emergency medicine management of decompression illness and arterial gas embolism.
In the United States, the prehospital emergency medical base station medical control is well worked out using the “911” entry into the hyperbaric medical system of the country by calling the Divers Alert Network (DAN) hot line. It is intended that HBOT be used in planned, controlled, and prospective human trials in stroke intervention in the United States. These stroke trials will utilize a similar “call 911 stroke” emergency medical service (EMS) telephone number. EMS HBOT therapy will be discussed further in the section on emergency medicine management of decompression illness and arterial gas embolism.
Promising yet investigational medical conditions which show improvement with the emergency use of hyperbaric oxygen are:
a) thrombolysis in acute myocardial infarct (see Chapter 23; Shandling 1997)
b) acute stroke (see Chapter 17)
c) acute head injury (see Chapter 19)
d) cardiopulmonary arrest
e) hanging (See Chapter 18)
f) near drowning (See Chapter18)
g) vascular headaches (see Chapter 22)
Tissue Ischemia as Common Factor For Emergency Medical Indications
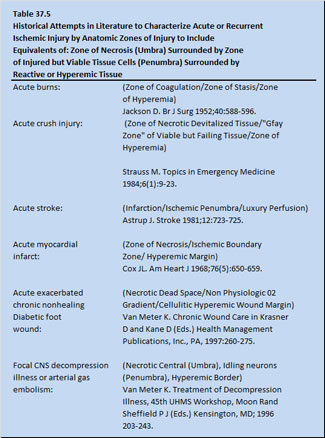 One possible common factor for many of the emergency medical indications is acute or recurrent tissue ischemia, the resuscitation of which initiates reperfusion injury in an anatomic construct of tissue injury zones. The anatomic construct of injury involves a zone of necrosis surrounded by a penumbral zone of injured but viable tissue surrounded by a zone of reactive hyperemia. Some of the past attempts to clarify injury pattern along these lines are shown in Table 37.5.
One possible common factor for many of the emergency medical indications is acute or recurrent tissue ischemia, the resuscitation of which initiates reperfusion injury in an anatomic construct of tissue injury zones. The anatomic construct of injury involves a zone of necrosis surrounded by a penumbral zone of injured but viable tissue surrounded by a zone of reactive hyperemia. Some of the past attempts to clarify injury pattern along these lines are shown in Table 37.5.
In summary, oxygen has a dose dependent effect in reduction of ischemic inflammation immediately in the post-injury period. Improving oxygen delivery to the zone of stasis (penumbra) supports or boosts viability of ischemic and dysfunctional but viable cells. Possibly, improved oxygen delivery to the zone of hyperemia (luxury perfusion) may suppress some of the inflammation in this zone of reaction to injury.
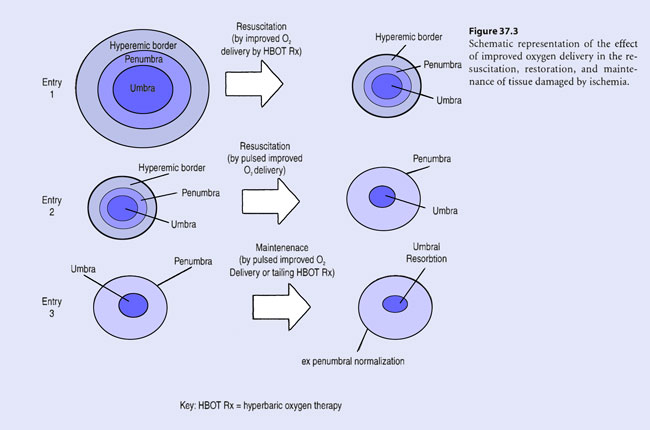
In convalescence, during recovery from injury, improved oxygen delivery aids in the resorption of the zone of coagulation (umbra) (see Figure 37.3, entry 3). Periodic pulsed administration of improved oxygen delivery induces wound contraction and soft tissue substitution into resorbing necrotic portions of ischemic wounds (a wound healing process termed “creeping”) (Van Meter 1997). This underlies the rationale for “tailing” hyperbaric treatments after initial resuscitative HBOT therapy in acute ischemic tissue injury.
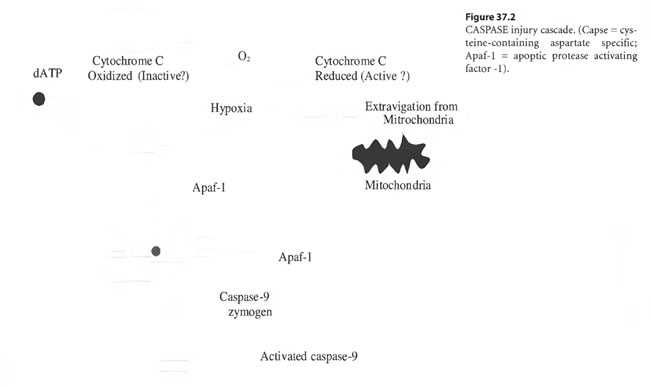
During convalescence, “tailing” hyperbaric treatments may induce continuing recovery in the acute penumbra as it converts to the “ex-penumbra.” Already, pulsed, improved oxygen delivery afforded by HBOT “tailing” treatments has been demonstrated to up-regulate m-RNA directed induction of platelet derived wound healing factor receptor sites in recovering ischemic tissue – the wound penumbra (Yu et al 1996; Zhao et al 1994). By pulsing wound penumbra with improved oxygen delivery, apoptosis may be limited by alteration of the redox potential of cytochrome C leached out of mitochondria into the cytosol. Oxidized cytochrome C may prevent the induction of the CASPASE injury cascade or arrest its progression whereas reduced cytochrome C may enhance or initiate it as shown in Figure 37.2 (Li 1997).
The effect of improved oxygen delivery in the resuscitation, restoration and maintenance of tissue damaged by ischemia is schematically represented in Figure 37.3.
In the past, the emergency medical use of HBOT has been described to effectively take advantage of Bigelowian flow of plasma through injured microvasculature stricken by endothelial derangement and occluded by formed blood element debris (Bigelow 1964). In a HBOT-treated patient, the plasma stream provides for adequate oxygen delivery to satisfy tissue oxygen demand (Boerema et al 1960) .
Various mechanisms of oxygen-dependent wound repair mechanisms that are facilitated by HBOT and described prior to 1985 are shown in Table 37.6. Effects of HBOT on the injured tissues of a patient are: (1) improves oxygen dependent tissue repair and (2) “medical decompression” of compartment syndromes. Since 1985, delivery of HBOT to patients with ischemic injured tissue has been based on the rationale shown in Table 37.7.
 Use of HBOT in Cardiopulmonary
Use of HBOT in Cardiopulmonary
Resuscitation (CPR)
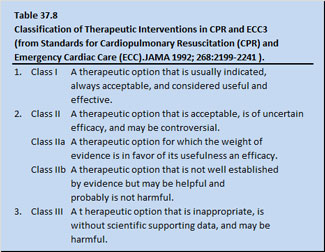
Oxygen is the primary drug in modern resuscitation of cardiopulmonary arrest patients. Supplemental oxygen should be used during cardiopulmonary emergencies as soon as it is available and in the highest possible concentration. The use of supplemental oxygen in resuscitation fits into a system of classifying recommendations in interventional resuscitation pharmacology proposed by the American Heart Association (Table 37.8).
Supplemental O2 in resuscitation is a Class I agent (a therapeutic option that is indicated, always acceptable, and is considered useful and effective). Classically, cardiopulmonary resuscitation of patients is done under normobaric conditions, allowing only a maximum of 100% oxygen to be administered. For humans, dose response curves for use of 100% oxygen at increased atmospheric pressure by hyperbaric exposure have not as yet been developed.
Currently, hyperbaric oxygen administered to a patient in cardiopulmonary arrest may be considered as a Class II (b) agent (a therapeutic option that is not well established by evidence, but may be helpful and probably is not harmful). Interestingly, one might wonder if a fraction of inspired oxygen at 100% (FIO2 100%) in normobaric conditions is a Class I agent, just how far along the way from a Class I agent on the way to a Class lib agent is a surface equivalent of inspired oxygen (in a hyperbaric environment) of 105% (SEFIO2 105%).
 Standard External Versus Open Chest CPR
Standard External Versus Open Chest CPR
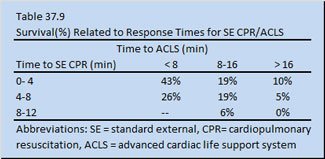
In the latter part of the 1950s modern standard external cardiopulmonary resuscitation (SE-CPR) and advanced cardiac life support (ACLS) emerged as a way to deliver oxygen to the mitochondria of a victim of cardiopulmonary arrest. Specifically, external defibrillation for ventricular fibrillation, mouth-to-mouth ventilation, and closed chest cardiac massage (or SE-CPR) merged into CPR/ACLS. Ironically, SE-CPR/ ACLS replaced open chest cardiac massage CPR (OC-CPR), which up until that point had provided the cardiopulmonary arrest patients a better outcome. Unfortunately, the combined SE-CPR/ ACLS approach to resuscitation has demonstrated no improvement in patient outcome over that achieved when it was first introduced over forty years ago. Resuscitation using SE-CPR is not as effective as that achieved with OC-CPR (Heller 1990) and resuscitation rates can be as low as 0 to 5% %(see Table 37.9).
Role of HBOT in CPR
Enhancement of patient oxygen delivery by hyperbaric oxygenation (HBOT) may be a productive way to implement better patient outcome as SE-CPR/ACLS is applied to cardiopulmonary arrest patients.
In CPR/ACLS, central venous oxygen content as measured indirectly by central venous 0₂ saturation, is receiving increased attention as an indicator of patient outcome (Snyder et al 1991).
Cardiopulmonary arrest patients with persistent central venous 0₂ saturations of less than 30% have no chance of recovery of spontaneous circulation and survival. All of cardiopulmonary arrest patients with a central venous oxygen saturation of greater than 72% recovered spontaneous circulation (Rivers et al 1992).
Tissue oxygenation is sensitive to changes in both convective and diffusive oxygen transport, which is not apparent under normal circumstances because oxygen is not present in excess (Douglas 1994). Cardiopulmonary arrest leads to hypoxia, which can be corrected by HBOT. Hyperbaric oxygen can produce central venous oxygen saturations of 100%, even in shock, and is capable of increasing both convective oxygen transport and diffusive oxygen transport.
Animal Experimental Studies
The role of HBOT in resuscitation was investigated in guinea pigs (Van Meter etal1988). Cardiac arrest was induced with intravenous injection of 2 cm3 of iced 1% KCl [Potassium Chloride] followed by 2 cm3 of iced normal saline concurrently with cross clamping tracheostomy tubes for 15 min. By continuous EKG monitoring, all animals were found to be asystolic or in fine ventricular fibrillation at 15 min. Cardiac arrest was induced in a hyperbaric chamber and resuscitation was done in the same chamber, whether it was pressurized or not. All animals had abdominal compression-chest compression CPR/ ACLS after 15 min of cardiopulmonary arrest. The animals were maintained at 34°C (± 1.5°C) by a heating pad servo-linked to a rectal temperature probe. Initially, during CPR/ACLS, all animals received 0.5 cm3 of 1/10,000 epinephrine intravenously administered by an internal jugular line and, thereafter, 0.25 cm3 of 1/10,000 epinephrine intravenously every 10 min.
Forty-two animals were divided into six groups for purposes of post-arrest resuscitation: three groups were ventilated during resuscitation with air at 1, 2.8, and 6 ATA, and three groups were ventilated with 100% oxygen at 1, 2.8, and 6 ATA. The animals were graded by success at initial post-resuscitation survival and mean post-resuscitation survival time. Results are shown in Table 37.10.
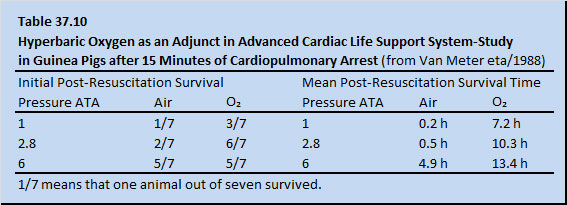
In all of the animals in the 6 ATA oxygen group and six of seven of the animals in the 6 ATA air group, asystole or fine ventricular fibrillation reverted to normal sinus rhythm rapidly on their way to compression. In all groups, whether compressed or non-compressed, the animals responded better to oxygen than to air at each respective pressure. Inhalation of pure oxygen itself is considered to be anti-arrhythmic. This work is now being conducted in both open chest and closed chest swine model in controlled, randomized, prospective trials at the Baromedical Research Institute (BRI) in New Orleans, and similar preliminary results are being achieved. The animals are being defibrillated by a human resuscitator at 1, 2, and 4 ATA while the animal is placed on oxygen. Perhaps, in the instance of the animals exposed to 4 ATA, oxygen diffuses through endocardial cardiac chamber surface into the ischemic cardiac conducting system during cardiopulmonary arrest. In this open chest swine model, return of spontaneous circulation is achievable after 25 min normothermic cardiopulmonary arrest with cardiac compressions, fixed low dose intravenous epinephrine and ventilation with SEFIO2 400%. Results of one published randomized, controlled animal trial are encouraging (Table 36.11).
In this study, initial success was defined as return of spontaneous circulation (ROS) with production of a mean arterial pressure (MAP) greater than 75 mmHg and sustained ROS at a MAP greater than 50 mmHg at 2 hours post resuscitation. Clearly resuscitation rates were better in the animals resuscitated at 4 ATA on oxygen than those resuscitated at either 1 or 2 ATA on oxygen. Brain lipid peroxidative change was likewise more ameliorated in the 4 ATA group (Van Meter 1999, 2001a).
Cardiopulmonary arrest upon resuscitation with return of spontaneous circulation imposes a global reperfusion injury upon a patient. HBOT therapy may immediately provide enough oxygen delivery to the cerebral and systemic circulation to attenuate biochemical injury cascades. There is theoretical concern that HBOT may aggravate the free radical mechanisms implicated in reperfusion injury, but this has not been substantiated. On reperfusion of ischemic tissue, hyperbaric oxygen has been demonstrated to lessen leukocyte endothelial adherence in microvasculature by modifying B₂ integrins (Thorn 1993b). Likewise, on reperfusion of ischemic tissue, HBOT, which is more effective than normobaric oxygen, promotes the conversion of xanthine oxidase to dehydrogenase (Thorn 1990). Oxygen provided to thrombosed microvasculature promotes endogenous thrombolysis by preventing the inactivation of leukocyte plasminogen activator by the plasma anti-plasminogen activator shield. Ample provision of oxygen to thrombosed microvasculature allows polymorphonuclear leukocytes to elaborate hypochlorous acid, which reacts with the methionine-rich plasma anti-tPA [Tissue Plasminogen Activator] shield protein. Leukocyte tPA, which is sparse in methionine, is spared (Weiss 1989). In simple terms, well-oxygenated thrombosed microvasculature undergoes endogenous thrombolysis more readily if the partially thrombosed microvasculature has an ample oxygen supply.
In animal experimental studies, HBOT has been demonstrated to accelerate neurologic recovery after cerebral ischemia (see Chapter 17). Likewise, hyperbaric oxygen alone and hyperbaric oxygen with tPA have been demonstrated to accelerate myocardial recovery in animal myocardial infarct models (see Chapter 23). Hyperbaric oxygen has been applied clinically in serial daily fashion to ameliorate central nervous system injury from ischemic insult as a post-injury “tailing” of treatments. Serial low-dose hyperbaric oxygen has been demonstrated to immunomodulate lymphocytes (see Chapter 24). Perhaps this use of hyperbaric oxygen will be applied to lessen the neurologic injury and improve the quality of life after successful resuscitation of patients suffering cardiopulmonary arrest in the future. Saving life is not the only goal of medicine; rather it is saving a life to be lived as a person (Miller 1993). Case 5 in the following section illustrates the use of HBOT as an adjunct to CPR in preventing neurological injury. Further experimental work in animals is needed, followed by controlled, randomized, prospective human trials. Institutional Review Board (IRB) approved consent for treatment in clinical resuscitation trials must respect the autonomy of the unresponsive patient. IRE-approved, presumed consent may be used provided that the previously FDA approved drug be used in a dose response trial. In the instance of oxygen in ACLS use, one solution would be to draw the choices together – for resuscitation oxygen breathing mix to be considered a Class lib agent closely approximating a Class I agent. For oxygen, this could be accomplished initially with a SEFIO2 of 110% by means of hyperbaric oxygen, compared with a FIO2 of 100% in normobaria, with presumed consent. If 110% SEFIO2 were found to improve patient outcome over and above FIO2 of 100%, then the patient dose response with presumed consent could be advanced to explore the advantage of a SEFIO2 of 120% compared with a SEFIO2 of 110%. Accordingly, a dose response curve for use of hyperbaric oxygen in SECPR/ACLS could be obtained, all with presumed consent. Animal experimental work substantiated preliminarily in acute focal ischemia of the CNS after stroke as well as in global ischemic insult of the CNS after cardiopulmonary resuscitation (Rosenthal et al 2003) that oxygen concentration is less injurious in the sequence arranged in order of least (1st) to most (3rd) reperfusion injury.
1st (3 ATA O2) SEFIO2 300%
2nd (1 ATA air) SEFIO2 21%
3rd (1 ATA O2) SEFIO2 100%
For evaluation and management of CPR/ACLS in the future it will be important to perform real-time monitoring of oxygen tissue delivery to obtain information for the servo-control FIO2administered during resuscitation of a victim of cardiopulmonary arrest. Clinically, transcutaneous p0₂ electrodes and intravenous fiber-optically connected optodes for oximetry have been used to evaluate human response to resuscitation of cardiopulmonary arrest. Optical spectroscopy also may play a role in non-invasively monitoring brain tissue oxygen delivery in CPR/ACLS, in an effort to develop more effective treatment of cardiopulmonary arrest.
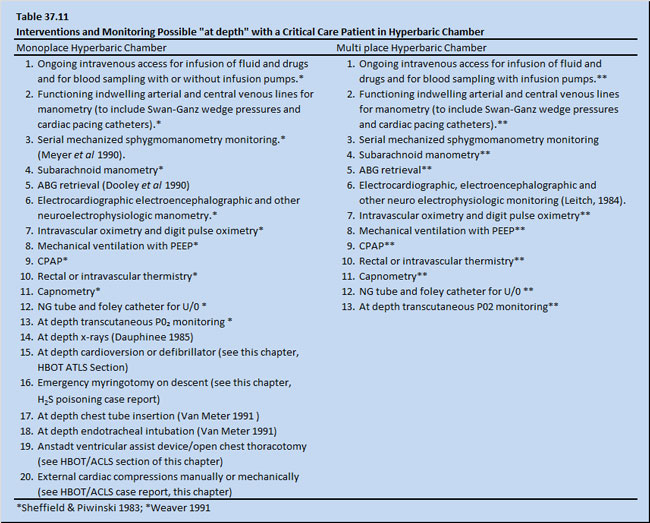
The hyperbaric chamber has been modified to allow conventional resuscitation of critically injured patients. Table 37.11 shows monitoring devices and interventions currently used routinely in both monoplace and multi place chambers (Weaver 1991; Moon & Camporesi 1991). It is now possible to reproduce in multi place or monoplace chambers all medical care and attentions necessary for critically ill or injured patients in a well-equipped emergency department.
Because all of the approved medical indications for both ACHM/UHMS listed previously in this chapter (see Table 37.4) have been thoroughly covered elsewhere in this textbook, details on the pathophysiology that makes them amenable to hyperbaric oxygen treatment will not be discussed in this chapter. Rather, seven cases will be presented to demonstrate the interaction of the emergency department with the prehospital EMS and the medical care: a diver affected with dysbarism, one case of severe CO intoxication, two chemists with severe H2S poisoning, one diver with acute blood loss from exsanguination from a duodenal ulcer arterial bleed, and one diver with severe arm crush injury and missed decompression,. An additional case of hyperbaric management of barotrauma and cardiopulmonary arrest is also presented.
Case Studies of Medical Emergencies Treated with HBOT
Prehospital EMS and Emergency Department Management of Decompression Illness/Arterial Gas Embolism (DCI/AGE)
Case 1: Prehospital EMS and Emergency Department Management of Decompression Illness/Arterial Gas Embolism (DCI/AGE)On June 16, 1996, a 60-year-old woman performed a single 60 feet sport SCUBA dive for 54 min off the west coast of Florida. She made an in-water stop of 15 feet for 4 min. She was a choreographer and dance team instructor and had maintained an excellent physical condition by jogging every day. She has no history of drinking, smoking or using recreational drugs. Shortly after surfacing, she experienced bilateral arm pain and hypesthesia caudally from the axillary level down. She had scapular pain and clonic jerks of her lower extremities which were easily elicitable. She was unable to stand due to weakness and instability. The boat from which she was diving had no available oxygen and her transport to land took 2 hours. Upon reaching land, while awaiting helicopter transport, paramedics placed her on oxygen by non-rebreathing mask with reservoir (O2 with NRM with R). She was transported for approximately 15 min at under 500 feet altitude to a hospital-based emergency department with a multiplace hyperbaric unit. Approximately three hours after surfacing, she was begun on a USN TT6 with O2 extensions. She had some improvement of scapular pain but no significant change in her condition. Approximately 24 hours later she was begun on a USN TT6A with O2 extensions with little improvement. Approximately 21 hours later she was begun on a USN TT6 with extensions, again with little improvement. The decision was made to transport her to a hospital based emergency department experienced with saturation hyperbaric oxygen treatment for refractory cases of DCI/AGE. She was transported by a Lear jet while on O2 with NRM with R by one atmosphere. At the new facility, the patient began a RN TT71 treatment table with nitrox 60/40 (nitrogen 60%/oxygen 40%) breathing mix at 165 feet sea water (fsw) at an approximate 52 hours after surfacing from her injurious dive. The chamber was injected with nitrogen to bring environmental O2 to a SEFIO2 of 35%. At 165 fsw after several nitrox 60/40 30 min breathing periods, her symptoms began to improve.
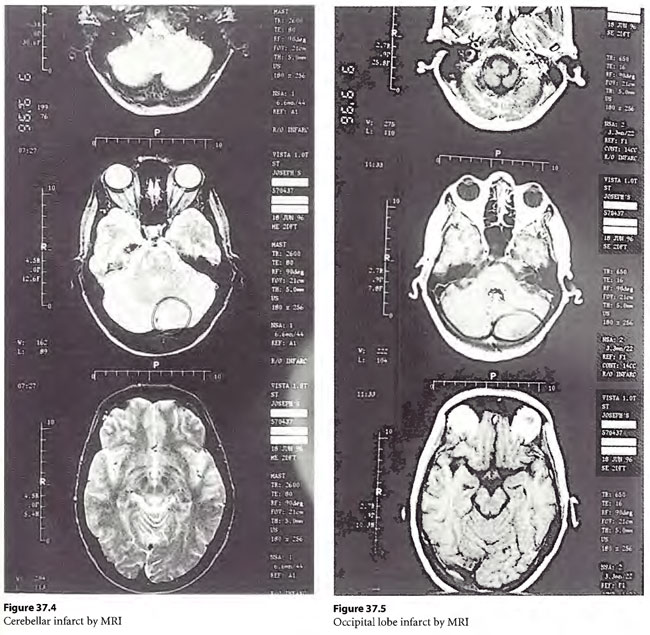
 The patient continued to improve and over the next 72 hours gradually came to surface on a RN 71 profile (the patient’s ascent was held at a stop each day between 1600 and 1800 and between 0000 and 0600) with intermittent nitrox 60/40 breathing periods deeper than 60 fsw and with 100% O2 breathing periods from 60 fsw to surface. She surfaced in an ambulatory and completely alert state. She was discovered by MRI to have an ischemic region in her cerebellum (Figure 37.4) and cortical occipital lobes (Figure 37.5). She was given six 33/90 tailing hyperbaric oxygen treatments and her unstable gait improved. Now, two years after this event, she runs and dances and has productively re-entered her choreographic career. She has no residual injury.
The patient continued to improve and over the next 72 hours gradually came to surface on a RN 71 profile (the patient’s ascent was held at a stop each day between 1600 and 1800 and between 0000 and 0600) with intermittent nitrox 60/40 breathing periods deeper than 60 fsw and with 100% O2 breathing periods from 60 fsw to surface. She surfaced in an ambulatory and completely alert state. She was discovered by MRI to have an ischemic region in her cerebellum (Figure 37.4) and cortical occipital lobes (Figure 37.5). She was given six 33/90 tailing hyperbaric oxygen treatments and her unstable gait improved. Now, two years after this event, she runs and dances and has productively re-entered her choreographic career. She has no residual injury.
Discussion
The patient exemplifies that all is not known that could be known about treatment of decompression illness and arterial gas embolism. Immediate post injury one atmosphere high concentration reoxygenation is advocated. The Divers Alert Network (DAN) in the United States urges the availability of high pressure oxygen flasks with oxygen administration equipment on all diving operations. It is not unusual to see even quite severe symptoms of DCI/ AGE ameliorate favorably with this early treatment approach. Cautious but complete hydration should be carried out orally if the patient is tolerant, but parenterally if the patient is not. The Divers Alert Network should be called for needed advice in the United States [(919)-684-2948] (or its equivalent in other countries). The patient should be transported to hyperbaric chamber for recompression and hyperbaric oxygenation. The prehospital essential emergency data set (EEDS) which should be conveyed to DAN and to the receiving emergency department should be along the lines shown in Table 37.12 and as advocated by Gorman (1990).
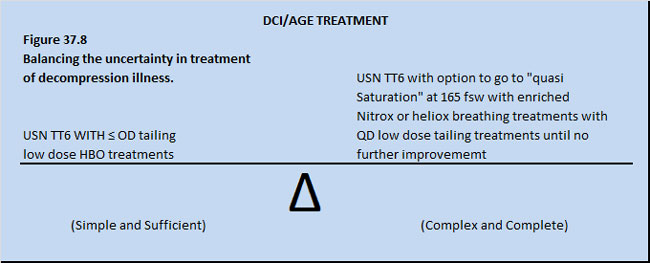
The ideal treatment table for human decompression illness and arterial gas embolism is not known. In fact, no human controlled, prospective, randomized trial exists to prove if the outcome of DCI or AGE patients is better if treated with or without HBOT, let alone with different hyperbaric treatment regimens (Flynn 1991). Further, no controlled, prospective, randomized trial exists to prove if DCI or AGE patients have a better outcome if treated with just a few ( < 5) or many(> 5) low dose tailing HBOT treatments after an initial definitive deeper HBOT treatment (Flynn, 1991). The uncertainty is summarized in Figure 37.8.
Several hyperbaric clinicians have reported cases where USN TT6’s did not resolve DCI/AGE injury, to be followed with resolution or improvement with a 165 fsw treatment table delayed as long as 72 hours after the “failed” USN TT6’s (Kol et al 1993a; Lee et al1991: Van Meter 1996). The 165 fsw tables incorporate enriched nitrox or heliox breathing periods at 165 fsw with stays often at 165 fsw of longer than 30 min with conservative decompression thereafter to surface. In our experience in New Orleans, the USN TT6A during decompression can itself cause DCI in the non-injured tender or recurrence of symptoms of DCI in the injured diver.
In fact, there is no firmly established time period beyond which hyperbaric treatment of residual injury from DCI/ AGE has definitely been found to be effective. It is not unusual to observe untreated symptoms present up to a week resolve by an initial recompression (Kizer 1982; Myers 1985). For all cases with residual neurologic injury after an initial recompression treatment for DCI/ AGE, the JESMC Department of Emergency Medicine and Hyperbaric Unit in New Orleans, Louisiana, has averaged 17 “tailing” hyperbaric oxygen treatments (Van Meter 1996) and the Duke University, F. G. Hall Laboratory’s Clinical Hyperbaric Unit in Durham, North Carolina, has averaged 13.7 “tailing” hyperbaric oxygen for similar cases (Davis et al 1994).
One thing is certain; no favorable O2 source should be bypassed in the push to have the patient recompressed. This includes surface O2 inhalation and monoplace chamber oxygen administration if multi place chambers are distant and transport to them would delay treatment.
Severe Carbon Monoxide Intoxication and Hyperthermia
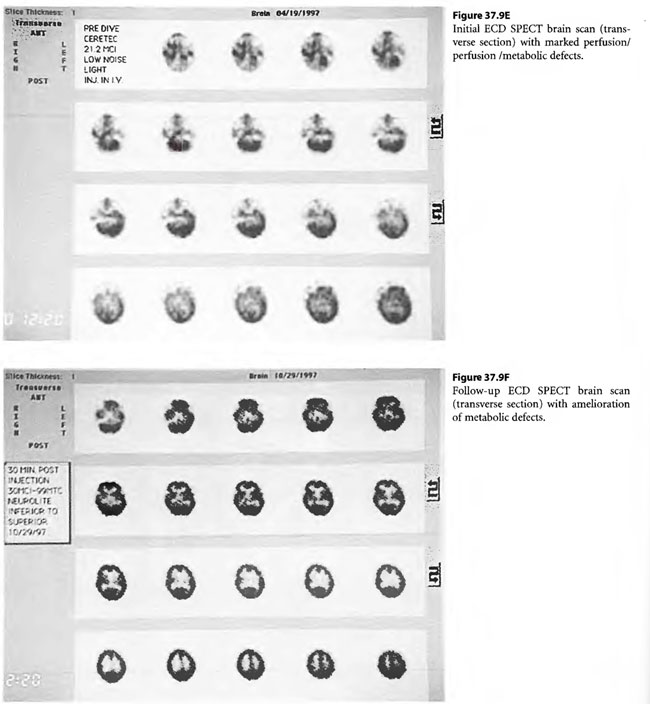
Case 2: Severe Carbon Monoxide Intoxication and Hyperthermia
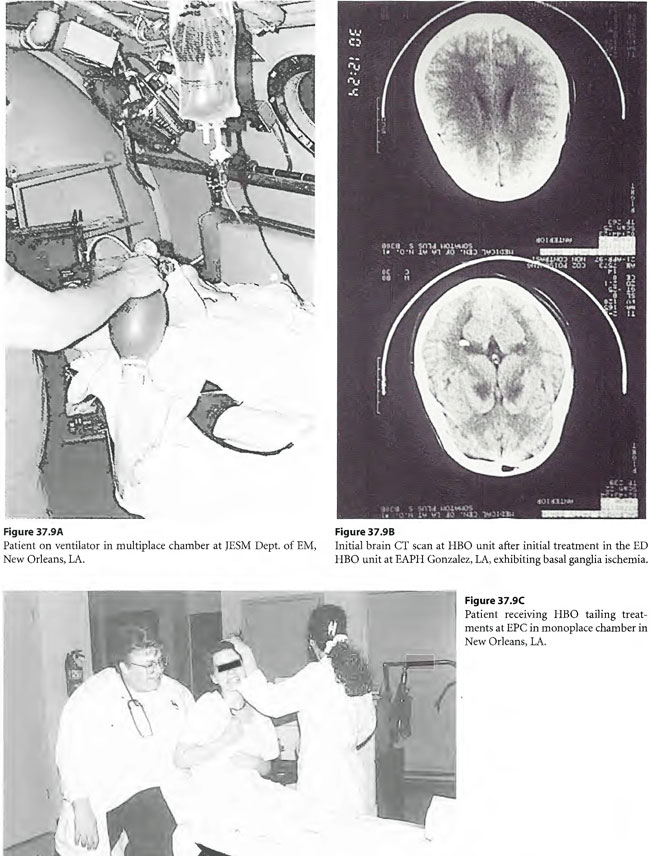
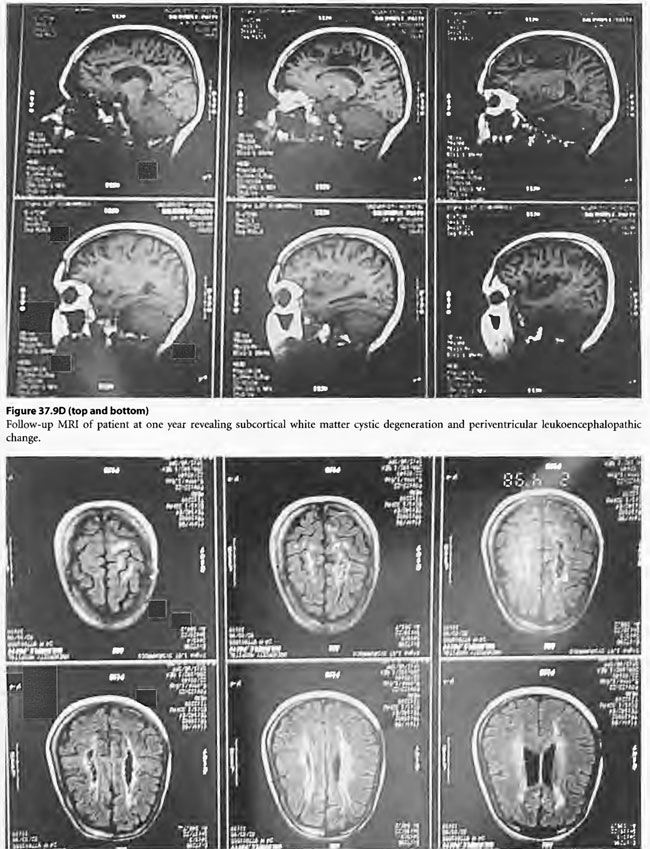
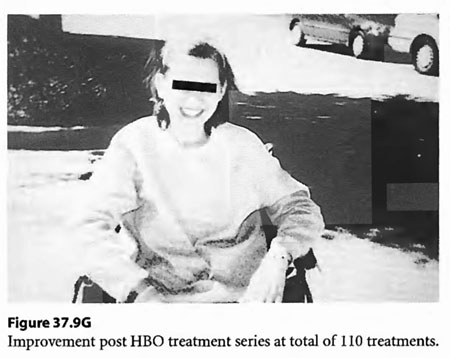
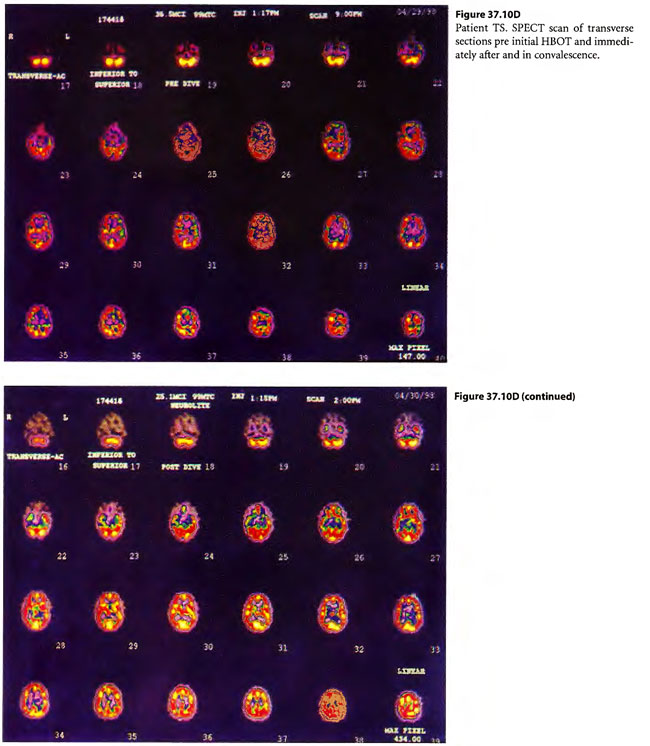
On April 6, 1997, a 22-year-old woman was found unconscious in a closed garage where the temperature was 130°F because of a housed running car. She was intubated by paramedics and brought in unconscious and hypotensive to the East Ascension Hospital Emergency Department in Gonzalez, Louisiana. She was then parenterally hydrated, cooled and given a HBOT treatment (modified monoplace USN TT6) while on ventilator. She then had a CT scan of the brain and was found to have ischemic change in the basal ganglia bilaterally. She was transferred in unresponsive coma to the Emergency Department at Charity Hospital (MCLNO) in New Orleans from where she was transferred across town to the Emergency Department at JESMC. There, she was treated while still on ventilator with a USN TT 6. She became more responsive at a depth at 60 fsw in the multiplace chamber at the JESMC ED but relapsed to coma at surface. She was weaned from the ventilator after 2 weeks. She was given a tailing series of 33/90 HBOT treatments four times daily for a total of 110 at no charge in a fully consented entry (her parents consented for her) in an IRB approved longitudinal case series for investigational use of HBOT therapy in severe brain injury. She was awake enough at depth on the 49th tailing hyperbaric treatment to say “stop!” out loud. She relapsed on surface but within a week was conversant with monosyllabic words and responses persisting at surface. She was quadriplegic (paresis of upper extremities and paraplegia of lower extremities) with contractures of the extremities. Over the next 61 treatments, she gradually began to move her limbs with return of strength as well as coordination and dissipation of contractures. She became conversant and recovered short-term memory. At the time of last examination she laughed and talked lucidly. She was able to feed and clothe herself and enjoys her two children. She took her first steps with the aid of a foot drop splint for her left lower extremity at exactly one year post- injury. This case is illustrated in Figure 37.9.
 Discussion
Discussion
Clinically, the optimal dose and duration of HBOT therapy after CO intoxication is not known. Anywhere from 8 to 20% of patients with severe CO intoxication develop neurologic sequelae (Grinker 1926). The expeditious use of HBOT therapy seems to limit the development of neurologic residual injury in some reported human trials. Goldfrank’s Textbook of Toxicologic Emergencies, the Bible in toxicology for most emergency physicians, currently states that, “although HBOT therapy cannot be recommended in every victim of carbon monoxide poisoning, it is a relatively safe treatment that should be considered in all serious exposures” (Tomaszewski 2002, Weaver et al 2002).
This patient’s tailing HBOT treatments stopped when her progress plateaued. Perhaps the patient’s penumbra areas of CNS injury were improved by the neurotrophic effect (on a signaling effect) of pulsed increased O2 delivery to her central nervous system afforded by HBOT therapy. In animal models subjected to both CO and hypotensive insult, HBOT has been demonstrated to truncate lipid peroxidation (Thorn & Elbuken 1991) and to prevent leukocyte adhesion in the CNS microvasculature (Thorn 1993b).
The period after the CO intoxication during which the residual injury remains receptive to amelioration by tailing HBOT therapy is not known. Reports in the literature indicate that the residual injury from CO intoxication improves with tailing low dose HBOT therapy even up to a year post-exposure in some cases (Van Meter et al 1994; Dean et al 1993; Neubauer 1979).
Severe Hydrogen Sulfide (H2S) Poisoning
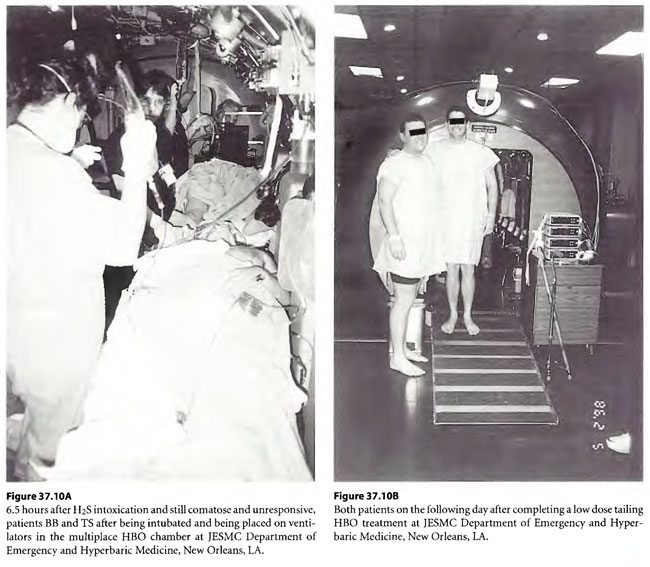
Cases 3 and 4: Severe H2S poisoning
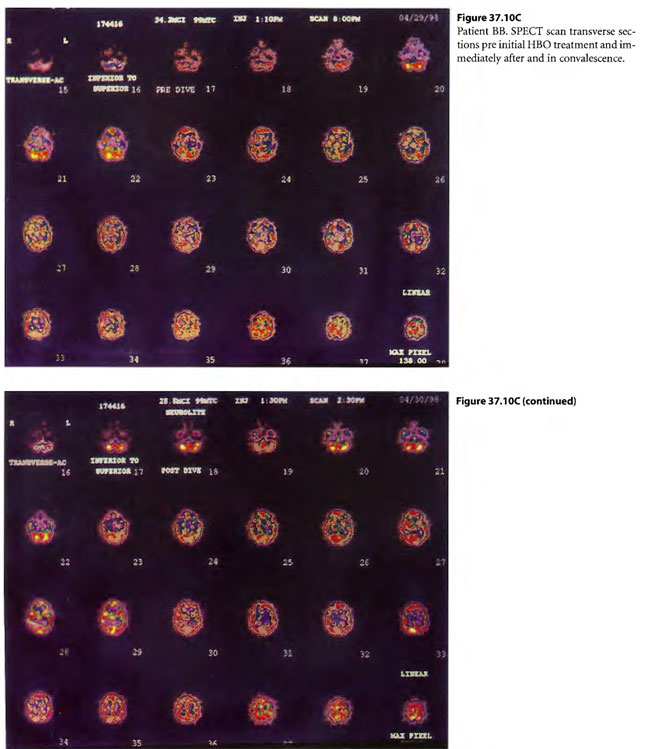
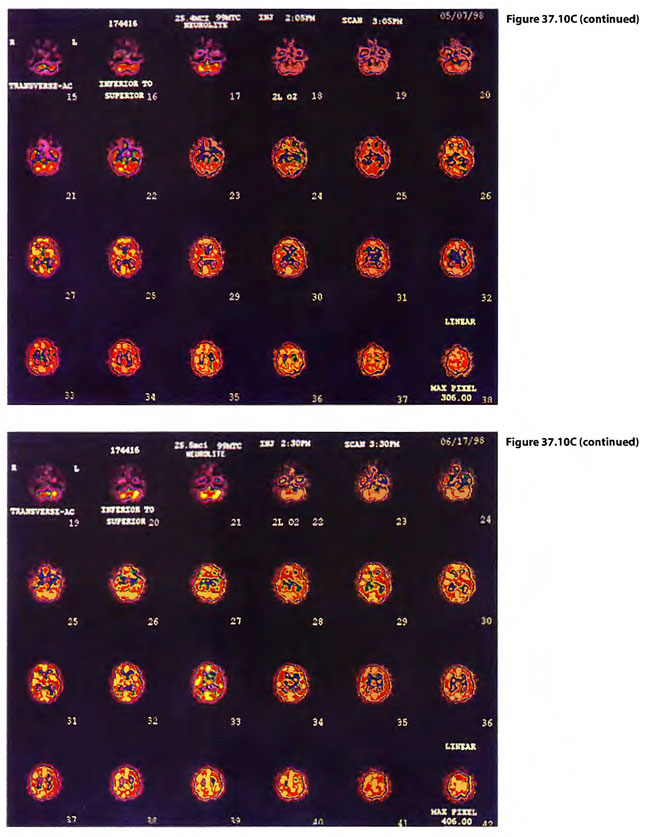
On 29 April 1998, a 29-year-old male chemical plant worker (BB) was adding pentasulfide pellets to alcohol in a “closed” hatching vat into which CO2 was being added to keep the exothermic reaction under control. A rotten egg odor was emitted and he ran from the vat, collapsing while running to land face first on a steel deck. He was apneic and unresponsive. A 32 year male co-worker (TS) came to his side to assist BB but collapsed on top of him. While the pressurized CO2 was cut off to the hatching vat, a third worker with self-contained breathing apparatus attempted mouth-to-mouth ventilation on BB after both BB and TS were pulled to safety. Paramedics placed the now breathing but comatose patients on O2 with NRM with R and transported the patients to the nearest hospital emergency department. TS began to have seizures shortly before arriving in the ED and BB began to have seizures shortly after arriving in the ED. Both were intubated by RSI and placed on mechanical ventilation with continuance of paralysis by pancuronium . The patients were given 300 mg of sodium nitrite (10 ml of 3% N2NO2 solution) to produce methemoglobinemia and were transported to the JESMC HBO2Emergency Department, New Orleans, where they were treated on a USN TT 6. Emergencies myringotomies were performed on both patients in the chamber on descent. During the second O2 period (still on ventilator) both patients woke up. The time from poisoning to the treatment time with the USN TT 6 was 5.6 hours. In the chamber, TS became more lucid, was cooperative and could be taken off ventilator, extubated, and put on a Duke hood. BB became combative and was re-sedated and re-paralyzed with pavulon and kept on ventilator. Four hours after the USN TT6, BB woke up in the ICU and was extubated. Both were treated on low doses (33/90 HBOT) treatments twice daily for following two days. BB was later diagnosed to have a perilymphatic fistula and had prophylactic pressure equalization tubes placed to allow further HBOT treatment. These low dose treatments were reduced to 45/60 BID but the patients reported feeling worse so the treatments were reduced to 33/90 four times daily where both of the patients reported improvement.
Post injury symptomatology included headaches, incoordination of gait and trouble concentrating. TS had clonus on ankle jerk on the left which disappeared by two weeks. While TS’s symptoms dissipated with time, BB’s symptoms persisted and BB was returned to a four times daily course of 0.15 mPa/60 min HBOT treatments tapered to once a day treatments. At this schedule gradual improvement was noticeable to the patient, his wife and the treating physicians. Cases 3 and 4 are illustrated in Figure 37.10.
Initially both patient’s C-spine x-rays and urine toxicologies were negative. (TS’s CT of the brain without contrast was negative and BB’s CT scan of the brain without contrast evidenced diffuse cerebral edema.) At the time of this writing, both patients have recovered completely.
Discussion
Goldfrank’s Textbook of Toxicologic Emergencies states: “The potential benefits of nitrite therapy and HBOT therapy should be considered for seriously ill patients exposed to hydrogen sulfide” (Kerns 2002). It is ironic that the standard therapy of production methemoglobinemia by administration of sodium nitrite (to produce a sulf-methemoglobin to pull H2S into inaccessibility from tissue mitochondria cytochrome liganding) in itself reduces hemoglobin’s ability to carry O2. In H2S poisoning, HBOT therapy may in part treat the decrement in O2 carriage caused by iatrogenic creation of methemoglobinemia.
While HBOT therapy has been used to effectively treat H2S poisoning experimentally in animals and incidentally in humans (case reports), the optimal dose or duration of HBOT therapy in H2S poisoning is not known (Whitecraft 1985; Smilkstein et al1985; Bitterman et al 1986). As with CO poisoning, HBOT therapy even when delayed, has been reported to lessen persisting neurologic symptoms by tailing of HBOT treatments (Pontani et al1998).
Hyperbaric Oxygen Therapy in the Use of Resuscitation
of Acute Bloood Loss Anemia

 Case 5: Use of HBOT in Resuscitation of Acute Blood Loss Anemia
Case 5: Use of HBOT in Resuscitation of Acute Blood Loss Anemia
On May 12, 2001, a 42-year-old commercial diver engaged in a saturation dive with storage at 660 foot sea water with working excursions to 750 feet sea water (Figure 37.11 and Figure 3 7.12). His recent routine medical diving clearance hemoglobin was 47 g/dL. Previously healthy, the diver was surprised to find himself especially fatigued on this operation. He was curiously short of breath without provocation. On more than one occasion, near syncopal episodes punctuated his work. In his storage dormitory chamber, on 22 May 2001, he diaphoresed, copiously stooled melena and syncoped. He was confused upon regaining consciousness. On 23 May 2001, in an increasingly confused and obtunded state, he again profusely diaphoresed and had massive hematemesis. A diver medical technician (DMT) locked down to the 660 fsw chamber depth to attempt intravenous access. The patient was placed on continuous 0.24 mPa O2 by mask (built-in breathing system [BIBS], which constituted a securely fitting oral-nasal mask with breathing gas scavaging system). The marked intravascular contraction in the patient disallowed successful intravenous access after an approximate 30 attempts by the DMT. The DMT could not obtain a blood pressure on the patient, but he could feel a feeble carotid rapid pulse. The bidirectional motility of the patient’s gastrointestinal tract precluded effective oral hydration. The DMT was instructed to implement extremity hypodermocleisis with normal saline at multiple extremity sites. This parenteral fluid loading method effectively returned the patient to a normotensive state with mild tachycardia. The normal saline was continued by hypodermocleisis short of producing clinically taught extremity compartment syndromes. The 0.24 mPa O2 breathing periods were administered by BIBS mask for periods of 20 min spaced by normoxic chamber atmosphere breathing periods (heliox with < 0.23 MB O2). These O2 breathing periods with normoxic breathing breaks were given in rounds of four titrated to relief of patient confusion or hypotension.
The patient began taking oral fluids with tolerance and retention. Next, oral hematenics (iron and B-complex vitamins), bouillon broth, and glucose supplement clear fluids were tolerated and retained. Routine decompression from storage depth to surface commenced as supplemented by the close interval cyclic 0.24 mPa O2 breathing periods before described. The diving support vessel made landfall. The patient walked out of the chamber upon surfacing, and land-based paramedics placed intravenous lines and placed him on precautionary supplemental oxygen for transport to an emergency department. There the patient had a hemoglobin of 4.7 g/dL and was transfused 6V2 matched units of packed red blood cells over time. The patient had diagnostic endoscopy to reveal a 3 mm duodenal ulcer which had eroded a small vessel which was now hemostatic. Helicobacter pylori sampling was positive. With oral antibiotic regime, the patient went on to completely resolve the duodenal ulcer as confirmed by endoscopic examination. The recovered patient is shown in Figure 37.13.
Discussion
In acute blood loss anemia, oxygen debt can be calculated by estimating a patient’s expected oxygen consumption minus calculated oxygen delivery over time.
1. Oxygen Content of Arterial Blood (CaO2): CaO2 = (grams Hg/ dL x 1.34 ml x 0% O2 Hgb) +(0.003 X mm pO2 )
(VanSlyke & Neill 1924)
2. Oxygen Delivery (DO2):
a. Cardiac Index (CI) = Cardiac Output (CO) ÷ Body Surface Area (BSA)
b. BSA =Square root of the quantity: height in inches x weight in pounds ÷ 3131
c. DO2 = CI x CaO2 (Chance & Chance 1988)
3. Oxygen Consumption (VO2): VO 2 = CO (CaO2 – CvO2) x time (Fick 1870)
4. Oxygen Debt= expected oxygen consumption over time or for a 2.8 m2 surface (56/h) -oxygen delivery over time (or
DO2)
Critical care specialists have established estimated predictors of clinical outcome in seriously injured or ill patients by calculation of patient oxygen debts in one atmospheric patient care settings. Patients may amass acutely reversible oxygen debt of 9 L/m2without sustaining residual organ injury. If a patient’s oxygen debt exceeds 22 L/m2, then multiorgan failure (MOF) is likely. If an oxygen debt of 33 L/m2 occurs, then death is likely (Shoemaker et al 1988).
Using the formulas above, the patient would have assuredly accumulated an oxygen debt in excess of 22 L/m2 (and perhaps even have exceeded 33 L/m2). The patient in fact recovered completely without any detectable CNS, myocardial, hepatic, intestinal, or renal residual injury. The patient went on to be a successful land-based housing renovation contractor.
More and more, hyperbaric oxygen therapy is being considered as an effective treatment modality to reduce oxygen deficit in acute illness or injury (Van Meter 2003).
Hyperbaric Oxygen Therapy in Severe Extremity Crush Injury
 Case 6: Use of HBOT in Severe Extremity Crush Injury
Case 6: Use of HBOT in Severe Extremity Crush Injury
On October 9, 2002, a 32-year-old commercial diver began a dive to 189 fsw with intended surface oxygen decompression to incorporate a deck decompression chamber. When the diver reached the Gulf bottom, he held on to the casing of a valve attached to the end of a long run of an underwater hydrocarbon pipeline. He opened the quarter turn ball valve in the valve casing and his right hand forcibly drew into the pipeline to his mid-forearm. The channel in the ball of the valve was 11h inches wide. In retrospect the pressurized hydrocarbon pipeline was at a lesser pressure than that of the 189 fsw pressure of the natural Gulf bottom. The relative vacuum in effect morphed the diver’s hand much like soft plastic stock pulled into a vacuum mold. His extremity was not degloved. Rather the skin envelope was disrupted only partly at the base of his thumb. His radius and ulna had a cross-section greater than that of the channel in the ball of the valve through which his hand and forearm were drawn. He aerobically exercised at great length and time to pull his arm out to free his left arm and to mitigate intense pain. A stand-by diver came down to assist him. A carborundom hydrolic rotary cutter could not be used to cut the pipe for concern of exploding the hydrocarbon content of the line. The stand-by diver used a hand hacksaw to arduously begin a cut through the thick schedule steel pipeline.
The base station diver medical service physician was advised of the problem when the injured diver had an approximate 75-min bottom time at 189 fsw. The choice to cut the diver’s arm or cut the pipe to free the diver to forestall the accumulation of disabling bottom time became an additional pressure for consideration in medical direction. No published U.S. Naval surface oxygen decompression air table existed for the diver’s depth or time at this point in the accident. Before the pipe was cut and the diver could be freed, 120 min of bottom time accumulated for the standby diver and 310 min of bottom time occurred for the injured diver. The water temperature was 40°F. The divers had unheated wet suits. Published U. S. Navy exceptional air exposure tables would have required 11 hours of in-water decompression for the stand-by diver and 15 hours of in-water decompression for the injured diver.
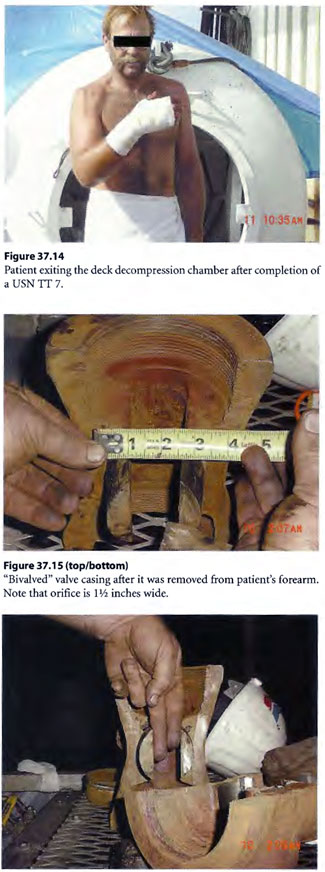
The New Orleans Emergency Decompression Rule (NO ED Rule) used often in the past for complicated decompression for cardiopulmonary resuscitation research dives and emergency treatment of seriously injured divers was utilized. The rule is explained in the discussion section of this case report. A fresh stand-by diver was sent down to assist the diver and free up the first stand-by diver. By use of the NO ED Rule, the first stand-by diver was first brought to surface as the “test case” to then finish necessary surface oxygen decompression with a USN Table 6 without problem. Next the freed up injured diver, still with cut off pipe and valve attached to his arm, was brought to surface utilizing the NO ED Rule for decompression profile. For life salvage, the patient needed surface oxygen decompression additionally. For limb salvage, the diver needed the valve casing cut off his hand and forearm. To accommodate this, the patient was put on a U. S. Navy Treatment Table 7 (USN TT 7) for required surface oxygen decompression and “tailing” HBOT treatment thereafter for the extremity crush injury (Figure 37.14). The USN TT 7 allowed ample air saturation soaking time to use mechanical cutters at depth in the chamber to arduously cut the valve casing off the diver’s arm (by “bivalving” the valve) in a compressed air environment rather than the nearly continuous oxygen breathing environment required by a USN TT 6 (Figure 37.15). The diver had distal extremity pulses intact after removal of the valve but had blisters (see Figure 3 7.16). The diver was up to date with tetanus toxoid immunization. Once in the deck decompression chamber, he was given a shot of ceftriaxone and oral doxycycline and oral levofloxacin. His blistered arm and forearm (referfusion injury) was copiously buttered with mupirocin ointment and dressed with cotton gauze strip dressing.
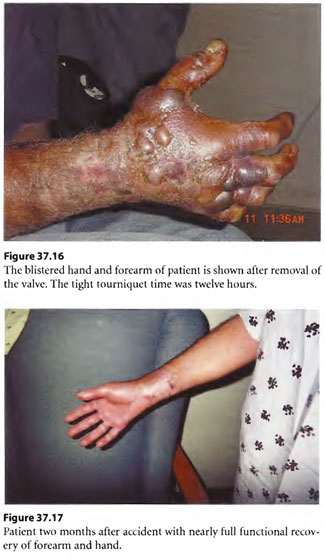 The diver came to surface and had serial low-dose (0.24 mPa/90 min) BID tailing HBOT treatments at a multiplace hyperbaric medicine unit. At two months post-accident, the diver had regained an approximate 80% mechanical function of his right extremity; at four months he was nearly completely recovered (Figure 37.17).
The diver came to surface and had serial low-dose (0.24 mPa/90 min) BID tailing HBOT treatments at a multiplace hyperbaric medicine unit. At two months post-accident, the diver had regained an approximate 80% mechanical function of his right extremity; at four months he was nearly completely recovered (Figure 37.17).
Discussion
At 189 fsw for a very prolonged bottom time, the uninjured stand-by diver and the injured diver faced life-threatening emergency decompression. No published surface supplied air surface oxygen decompression tables existed at the time of this accident. The 40°F water temperature and injury level for the patient made the U. S. Navy exceptional air exposure decompression tables very dangerous.
The NO ED Rule mentioned earlier allowed the use of published US Navy heliox surface oxygen decompression tables. These tables were supplemented by padded decompression to address the extreme cold exposure with marked physical exhaustion present. The NO ED Rule also addresses the inapplicability of applying heliox decompression to an air dive.
Specifically, the NO ED Rule allows padding of published tables by adding on to the table to be used 2 [units of 10 fsw to depth] plus 2 [2 of 10-min units of bottom time] and/or supplemental in-water oxygen decompression further supplemented by a USN TT 6 for surface oxygen decompression. Specifically each 10-min time or 10-foot depth overrun can be substituted by nitrox 60/40 breath on water stops by adding on one deeper water stop supplemental nitrox breathing before the 50 fsw in-water oxygen breathing water stop.
The USN heliox table applied to both the stand-by diver and the injured diver as modified by the NO ED Rule is given in Tables 37.13 and 37.14, respectively.
The rule allows for substituted decompression elements to be added if published tables do not extend to required depths of all bottom times. For the stand-by diver, in lieu of having published table times that went beyond 120 min, a 140 min bottom time would be achieved by adding 60/40 nitrox breathing at two in-water decompression stops deeper than 50 fsw. In the instance of the injured diver, the bottom time exceeded the maximum allowable published bottom time of 120 min (the 120 min published fell short of the actual bottom time of 310 min by 70 min). 20 min thereof were converted to 20 feet in theoretically added padded depth and 50 min were padded by nitrox 60/40 breathing on at least 5 in-water stops deeper than 60 fsw.

 It is noted that the USN heliox surface oxygen decompression tables incorporate oxygen breathing on in-water stops at 50 and 40 fsw remained unchanged.
It is noted that the USN heliox surface oxygen decompression tables incorporate oxygen breathing on in-water stops at 50 and 40 fsw remained unchanged.
The rule has been checked by one of the acknowledged contributors (Vasquez 2003) by the use of the NOBENDEM (US Naval Diving Manual Volume 2 and 3, revision 4, March 2001) computer program and Zwart’s Advanced Decompression Tables. This approach has been established to provide safe, sufficient, and not excessive decompression in emergency decompression for unanticipated prolonged bottom times on air dives.
Hyperbaric Oxygen Therapy as an Adjunct to CPR Following Barotrauma
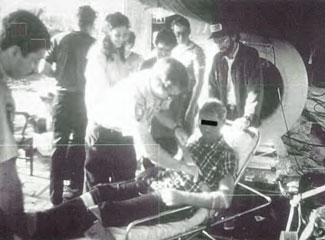
Case 7: Use of HBOT as an Adjunct to CPR Following Barotrauma
RP, a 35-year-old commercial male diver, dove on surface supplied air to 83 fsw for 25 min in the Mississippi River in approximate 29°C. His task was to airlift sand off a sunken river barge. His work was brought abruptly to a halt when his helmet was accidentally sucked into the intake of the airlift. His DESCO® hard helmet became caught on a compressed air injector elbow joint inside the intake orifice of the airlift, preventing him from being sucked in entirety up the airlift conduit. The airlift applied a forceful differential suction to the helmet neck dam of his “diving dress,” and severely barotraumatized his head and neck. He opened fully the free-flow air supply valve to his helmet.
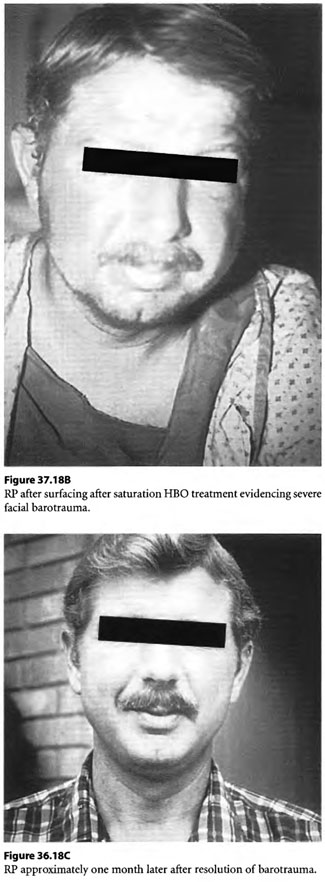 The oronasal mask within his helmet rippled “in the wind” of the high velocity air supply that air compressors topside were forcing down his umbilical air supply line to keep up with the relative vacuum that was produced by disruption of the seal of his neck dam by the airlift. In the rarified air, he became hypoxic and lost consciousness. Topside crews shut off the airlift and he was brought to the surface by a stand-by diver. On the surface, on a working support barge, his helmet was removed. His edematous, ecchymotic head was barotraumatized sufficiently to hide the location of his mouth. The patient was not breathing and a pulse could not be obtained as he was placed supine in upright position on the inner circumference of a small deck decompression chamber. Chest compressions without mouth-to-mouth ventilation were begun and he slowly descended to 165 fsw on air at which depth he had an oxygen supply mask (built-in breathing system mask without overboard dump) strapped to his face over what was assumed to be his mouth. Chest compressions without ventilation were continued. He received a surface equivalent fraction of inhaled oxygen (SEFIO2) of 600% for 10 min before he woke up to pull off the oxygen mask himself. Some 22 min elapsed from the time he was sucked into the airlift until the time he had CPR with O2 administration. The injured diver was ascended with periodic HBOT breathing periods of SEFIO2 of 300% on a treatment table which would resemble the USN TT 8 developed years later. It was noted that the diver’s swollen, ecchymotic head and neck appeared purple/black during air breaks but during hyperbaric oxygen breathing periods with SEFIO2 of 300% his skin would turn tomato red as the extravasated dermal extravascular hemoglobin would become saturated with oxygen.
The oronasal mask within his helmet rippled “in the wind” of the high velocity air supply that air compressors topside were forcing down his umbilical air supply line to keep up with the relative vacuum that was produced by disruption of the seal of his neck dam by the airlift. In the rarified air, he became hypoxic and lost consciousness. Topside crews shut off the airlift and he was brought to the surface by a stand-by diver. On the surface, on a working support barge, his helmet was removed. His edematous, ecchymotic head was barotraumatized sufficiently to hide the location of his mouth. The patient was not breathing and a pulse could not be obtained as he was placed supine in upright position on the inner circumference of a small deck decompression chamber. Chest compressions without mouth-to-mouth ventilation were begun and he slowly descended to 165 fsw on air at which depth he had an oxygen supply mask (built-in breathing system mask without overboard dump) strapped to his face over what was assumed to be his mouth. Chest compressions without ventilation were continued. He received a surface equivalent fraction of inhaled oxygen (SEFIO2) of 600% for 10 min before he woke up to pull off the oxygen mask himself. Some 22 min elapsed from the time he was sucked into the airlift until the time he had CPR with O2 administration. The injured diver was ascended with periodic HBOT breathing periods of SEFIO2 of 300% on a treatment table which would resemble the USN TT 8 developed years later. It was noted that the diver’s swollen, ecchymotic head and neck appeared purple/black during air breaks but during hyperbaric oxygen breathing periods with SEFIO2 of 300% his skin would turn tomato red as the extravasated dermal extravascular hemoglobin would become saturated with oxygen.
The diver made an uneventful recovery after therapeutic air saturation decompression supplemented by enriched nitrox breathing periods. Neurologic examination, as well as psychometric and neuroelectrophysiologic testing upon exiting the chamber, did not reveal any abnormalities. The case is illustrated in Figure 37.18.
Potential Future Uses of HBOT Therapy in Emergency Medicine
As mentioned earlier in this chapter, HBOT therapy has had both promising animal and human controlled randomized trials reported in the literature which indicate improvement with thrombolysis and HBOT therapy in acute myocardial infarct (Thomas et al 1990; Shandling et al 1997 ). Likewise, hangings (Mathieu et al 1987) and near drowning (Shn-Rong 1995) have had encouraging responses to HBOT, as shown in published human case reports.
Multicenter controlled prospective randomized trials of HBOT therapy in hyperacute stroke are planned (see Chapter 17). A future arm of the trial may even have paramedics enroll patients at the site of their ictus in the community by response with a hyperbaric chamber aboard an ambulance. The concept of having immediate HBOT in the prehospital phase using van or ambulance transportable hyperbaric chamber is not so new. The French proposed the same in the late 1800s in Paris to address an epidemic of CO Poisonings (Bert 1878) and the British in the 1960s used a Vickers monoplace chamber aboard an ambulance to resuscitate acute myocardial infarct patients in transport to the hospital (Smith 1962; Moon et al 1964). The Paracel transport chamber, the Drager transportable treatment chamber, and, more recently, the inflatable transport chamber have become shelf items that could be transported by land or air ambulances for the EMS (Van Meter et al 1992; Van Meter et al 1999).
The Academic Emergency Medicine Committee Task Force on prehospital management of stroke has recently published a recommendation that patients be given “low flow” oxygen in the field instead of higher concentrations of FIO2 of 100% achievable by high flow mask administration (Pepe et al 1998). This reflects the clinical intrigue we face as we progress to the next century to unravel the oxygen dosing paradoxes in emergency medicine in an attempt to improve our patients’ outcome in illness and injury.
Acknowledgments
We would like to acknowledge the Baromedical Research Institute in New Orleans, LA and our colleagues Diana Barratt, MD, James Moises, M.D., Paul Staab, M.D., Heather Murphy-Lavoie, MD, and Fred Kriedt, Ph.D. We also wish to acknowledge Johnny Vazquez, M.D. and Julie Anderson, M.D. for their evaluation and assistance in the write-up of the crush injury and acute blood loss case reports. We also wish to express our appreciation to Stephanie Dudenhefer and Sylvia Cusimano for their assistance in preparation of this manuscript.